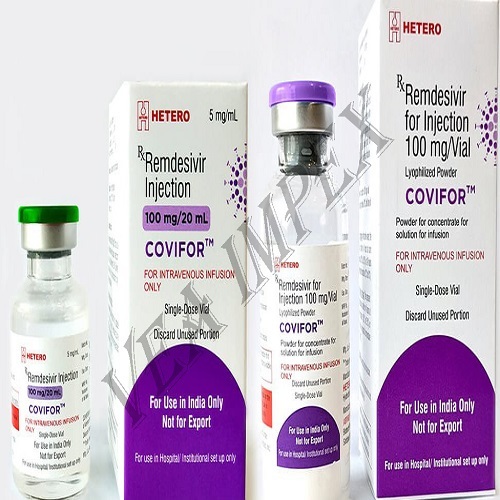
Remdesivir injection
10 - 10000 USD ($)/Piece
Product Details:
- Drug Type Injection
- Physical Form Tablets
- Function Other
- Suitable For Suitable For All
- Quantity 1 Boxes
- Click to view more
X
Remdesivir injection Price And Quantity
- 5000-1000 Piece
- 10 - 10000 USD ($)/Piece
- 10.00 - 100.00 USD ($)/Piece
Remdesivir injection Product Specifications
- Other
- Tablets
- Injection
- Suitable For All
- 1 Boxes
Remdesivir injection Trade Information
- Nhava Sheva
- 10000 Piece Per Week
- 7-15 Days
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- As per customer request
- Australia North America South America Eastern Europe Western Europe Middle East Africa Asia Central America
- All India
- WHO,GMP
Product Description
Uses
Remdesivir is an investigational drug being studied to treat coronavirus infection, also known as COVID-19. It has not been approved by the FDA for general use. However, the FDA is now allow ingremdesivir to be used in human studies and for emergency use in certain hospitalized patients. More information should be provided by the doctor from either the Informed Consent form for a study or the FDA Fact Sheet for Patient sand Parents/Caregivers for emergency use.
How to use Remdesivir 100 Mg Intravenous Powder For Solution (EUA)
Remdesivir is given by injection into a vein usually once daily for 5 to 10 days. It is infused over a period ranging from 30 minutes to 2 hours.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'COVID - 19 Medicine & Products' category
"We mainly deliver our products to the foreign countries."

 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese